Helicobacter pylori
Download / Print Section as PDFThe second Irish Helicobacter pylori Working Group consensus for the diagnosis and treatment of Helicobacter pylori infection in adult patients in Ireland
Introduction
Methods
Data review, assessment of evidence and revision of consensus statements
Recommendations
Domain 1: diagnosis of Helicobacter pylori infection in symptomatic adults
Statement 1: all patients with symptoms related to the upper gastrointestinal tract should be tested for Helicobacter pylori
Table 1. Criteria for assessing data quality and the strength of recommendations
|
Criteria for assessing data quality and the strength of
recommendations
|
|
Quality of evidence
|
|
Hight - Further research is very unlikely to change confidence in the estimate of the effect. Moderate - Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. Low - Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
|
Strength of recommendation
|
|
Strong - Strong recommendation for using an intervention. Strong recommendation against using an intervention.
Weak - Weak recommendation for using an intervention. Weak recommendation against using an intervention. |
Statement 2: the urea breath test is the recommended noninvasive test for Helicobacter pylori. If the urea breath test is unavailable, the monoclonal stool antigen test is an alternative noninvasive test if locally validated
|
Table 2.
Summary of consensus recommendations
|
|
Statements Strength Quality
|
|
Diagnosis of H. pylori infection in symptomatic adults
|
|
Statement 1
Strong High
All patients with symptoms related to the upper gastrointestinal tract should be tested for H. pylori.
Statement 2
Strong High
The UBT is the recommended noninvasive test for H. pylori. If the UBT is unavailable, the monoclonal stool antigen test is an alternative noninvasive test if locally validated.
Statement 3
Strong High
A combination of histology, taken from the antrum and corpus, and a RUT are recommended for invasive H. pylori testing.
Statement 4
Strong Moderate
A corpus and antrum biopsy sample should be taken for the RUT.
Statement 5
Strong Moderate
If H. pylori cultures are required, a corpus and an antrum biopsy should be taken.
Statement 6
Strong Moderate
AST by culture or a locally validated molecular method should be performed for national resistance surveillance and prior to prescribing clarithromycin-containing first-line therapy.
Statement 7
Strong High
Posteradication treatment testing must be performed. If gastroscopy is not required, a UBT is recommended for posteradication treatment testing. If the UBT is unavailable, the monoclonal stool antigen test is an alternative if locally validated.
Statement 8
Strong High
PPIs significantly reduce the accuracy of the recommended H. pylori tests; therefore, PPIs should be stopped 14 days before testing unless PCR testing is available.
|
|
First-line H. pylori therapy
|
|
Statement 9
Strong Moderate
14-day clarithromycin-based triple therapy with a high-dose PPI can only be recommended in cases where clarithromycin susceptibility has been confirmed.
Statement 10
Strong Moderate
Bismuth quadruple therapy with a high-dose PPI, metronidazole and tetracycline for 14 days is the recommended first-line treatment in the absence of clarithromycin susceptibility testing or where clarithromycin resistance has been confirmed. Although inferior eradication rates have been reported, doxycycline may be used if tetracycline is unavailable.
|
|
Second-line and rescue therapy
|
|
Statement 11
Strong Low
Bismuth quadruple therapy with a high-dose PPI, levofloxacin and amoxicillin for 14 days is the recommended second-line treatment. For those who received AST-guided clarithromycin first-line therapy a combination of PPI, bismuth, metronidazole and tetracycline can be given.
Statement 12
Strong Low
14-day rifabutin, amoxicillin triple therapy with high-dose PPI is the recommended rescue therapy. Alternatively, a combination of high-dose PPI and bismuth with 2 antibiotics not previously prescribed can be used.
Statement 13
Strong Low
For those with persistent H. pylori infection following unsuccessful eradication attempts, there is no current evidence-based guideline on appropriate follow-up. The IHPWG consensus is that endoscopic surveillance with ESGE-recommended mapping biopsies for precancerous changes should be considered at an interval of 5–10 years from last failed treatment.
Statement 14
Strong Moderate
As part of a quality assurance programme, H. pylori first-line, second-line and rescue therapy eradication rates should be locally monitored and audited as part of national and European registries.
|
Statement 3: a combination of histology, taken from the antrum and corpus, and an rapid urease test are recommended for invasive Helicobacter pylori testing
Statement 4: a corpus and antrum biopsy sample should be taken for the rapid urease test
Statement 5: if Helicobacter pylori cultures are required, a corpus and an antrum biopsy should be taken
Statement 6: antimicrobial susceptibility testing by culture or a locally validated molecular method should be performed for national resistance surveillance and before prescribing clarithromycin-containing first-line therapy
Statement 7: posteradication treatment testing must be performed. If gastroscopy is not required, a urea breath test is recommended for posteradication treatment testing. If the urea breath test is unavailable, the monoclonal stool antigen test is an alternative if locally validated
Statement 8: proton pump inhibitors significantly reduce the accuracy of the recommended H. pylori tests; therefore, proton pump inhibitors should be stopped 14 days before testing unless PCR testing is available
Domain 2: first-line treatment of Helicobacter pylori infection
Statement 9: 14-day clarithromycin-based triple therapy with a high-dose proton pump inhibitor can only be recommended in cases where clarithromycin susceptibility has been confirmed
|
Table 3.
Recommended treatment descriptions
|
|
Treatment regimen Description Duration
|
|
Clarithromycin PPl
a
b.i.d. 14 days 500 mg clarithromycin b.i.d
amoxicillin 1g amoxicillin b.i.d
triple therapy |
Statement 10: bismuth quadruple therapy with a high-dose proton pump inhibitor, metronidazole and tetracycline for 14 days is the recommended first-line treatment in the absence of clarithromycin susceptibility testing or where clarithromycin resistance has been confirmed. Although inferior eradication rates have been reported, doxycycline may be used if tetracycline is
unavailable
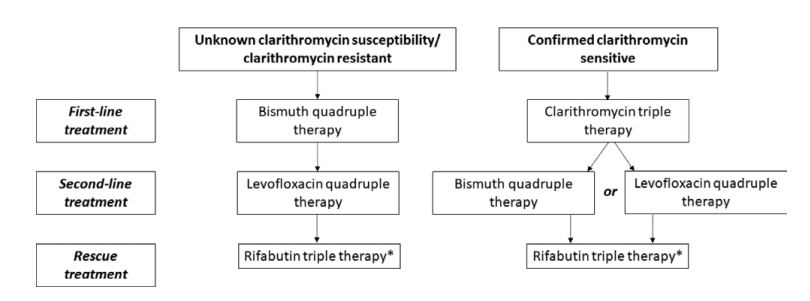
Domain 3: second-line and rescue therapy for Helicobacter pylori infection
Statement 11: bismuth quadruple therapy with a high-dose proton pump inhibitor, levofloxacin and amoxicillin for 14 days is the recommended second-line treatment. For those who received antimicrobial susceptibility testing-guided clarithromycin first-line therapy, a combination of proton pump inhibitor, bismuth, metronidazole and tetracycline can be given
Statement 12: 14-day rifabutin, amoxicillin triple therapy with high-dose proton pump inhibitor is the recommended rescue therapy. Alternatively, a combination of high-dose proton pump inhibitor and bismuth with two antibiotics not previously prescribed can be used
Statement 13: for those with persistent Helicobacter pylori infection following unsuccessful eradication attempts, there is no current evidence-based guideline on appropriate follow-up. The IHPWG consensus is that endoscopic surveillance with ESGE-recommended mapping biopsies for precancerous changes should be considered at an interval of 5-10 years from last failed treatment
Statement 14: as part of a quality assurance programme, Helicobacter pylori first-line, second-line and rescue therapy eradication rates should be locally monitored and audited as part of national and European registries
Concluding remarks
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References